Top seller
Endura Competent Cells
Product Details
ShowEndura™ Competent Cells include Control DNA and Recovery Medium, and are packaged as SOLOs (1 transformation per tube) or DUOs (2 transformations per tube) as indicated. Endura Electrocompetent cells are provided in DUOs format only. Recovery Medium is also available separately. The specified transformation efficiencies are with pUC DNA, unless indicated otherwise.
Endura Competent Cells
Key features
Show- Clone repetitive sequences and lentiviral libraries
- Stabilise direct repeats and create lentiviral constructs
- Generate CRISPR-GeCKO-Libraries-Endura lentiviral guide RNA libraries
- Recommended in CRISPR GeCKO library protocols
- Choose electrocompetent or chemically competent cells
- Highest efficiency commercially available cells for lentiviral cloning
Product information
Maintain unstable DNA.
Endura Competent Cells are a commonly used strain for cloning sequences that suffer unwanted recombination events in other strains. Clones with inverted repeats or other sequences prone to recombination are commonly found in retroviral genes, and require cells such as Endura to be propagated stably.Excellent value and efficiency! Switch to Endura cells today and experience a higher level of efficiency and a savings in your cloning budget.
Chemical or Electrocompetent. Whichever method of transformation you prefer, we can give you better efficiency and/or better prices.
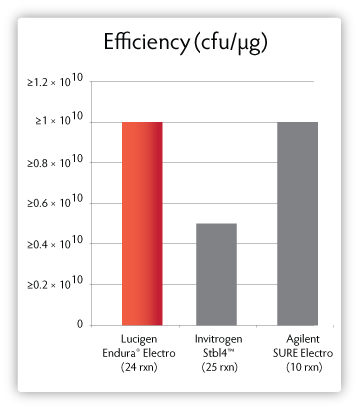
Figure 2. Transformation efficiencies of electrocompetent clone-stabilising strains.
Genotype:
recA13 supE44 ara-14 galK2 lacY1 proA2 rpsL20(StrR) xyl-5 λ– leu mtl-1 F– mcrB mrr hsdS20(rB–, mB–)
SDS
-
60240 Endura Chemically Competent Cells Duo
-
60242 Endura ElectroCompetent Cells
-
60241 Endura Chemically Competent Cells Solo
Manuals and user guides
Product information sheets
Access support
Need some support with placing an order, setting up an account, or finding the right protocol?
Contact us