LavaLAMP RNA Master Mix
Product Details
ShowBoth LavaLAMP RNA Master Mix kits contain: LavaLAMP RNA Master Mix, RNA Positive Control LAMP Primer Mix, and RNA Positive Control. The LavaLAMP RNA Master Mix with Dye also contains Green Fluorescent Dye for fluorescent detection of amplified DNA. The Green Fluorescent Dye is also available separately.
LavaLAMP RNA Master Mix
Key features
Show- Isothermal Amplification: Facilitates running amplification reactions with simplified instrumentation
- Master Mix Format: Streamlines reaction setup while reducing potential handling errors
- Minimal Optimisation: Focuses optimisation on the two critical reaction parameters - primer design and reaction temperature
- Lyophilisation-ready: Avoids redesign of assays to remove components known to inhibit lyophilisation all components are lyophilisation-compatible
Product information
The LavaLAMP RNA Master Mix is built around a proprietary DNA polymerase with strand displacement activity that works on both RNA and DNA templates. These enzyme features make it possible to perform RNA LAMP (loop-mediate isothermal amplification starting with RNA targets/templates) in which the RNA target is first converted into cDNA and then that cDNA acts as template for additional LAMP, all using a single DNA polymerase. By combining the strong activity of this enzyme with an optimised master mix formulation, we’ve produced an easy-to-use system that enables fast, sensitive detection of specific RNA targets.
Most LAMP enzymes are available as multi-component kits that require optimisation of many parameters such as MgSO4, betaine, and enzyme concentrations as well as reaction temperature, primer design and primer concentration. The LavaLAMP RNA Master Mix greatly simplifies reaction optimisation by providing a “pre-optimised reaction formulation” allowing you to focus your optimisation on the two most important reaction parameters – RNA LAMP primer design and reaction temperature.
Simplified Reaction Set Up and Optimisation with a Master Mix
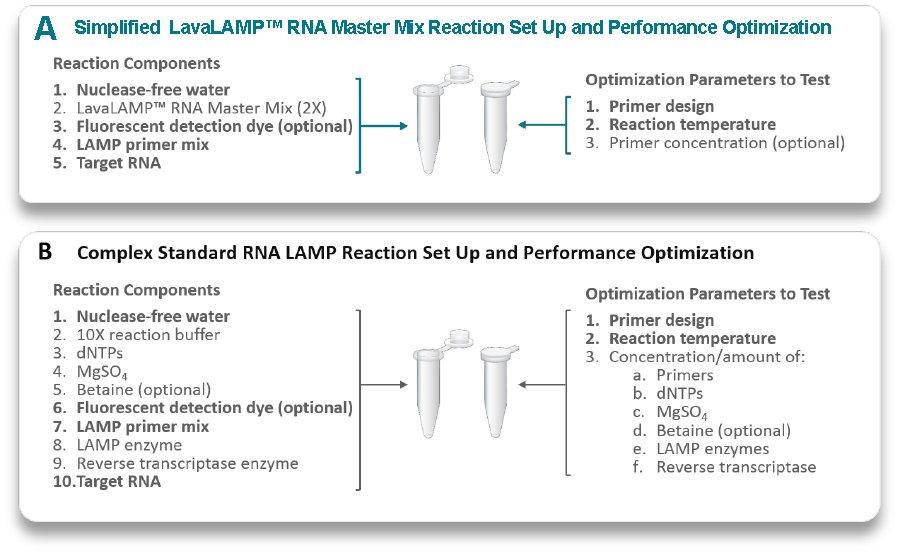
Figure 1. The differences in RNA LAMP reaction set up and potential optimisation parameters when using a master mix vs. a component kit format. Panel A illustrates the components added to a RNA LAMP reaction (left) when using the LavaLAMP RNA Master Mix and the various parameters than can be optimised (right). Panel B illustrates reaction setup (left) and potential optimisation parameters (right) when using a standard RNA LAMP component kit. Bold text indicates components or parameters common to both systems.
Quicker RNA Detection with LavaLAMP RNA Master Mix
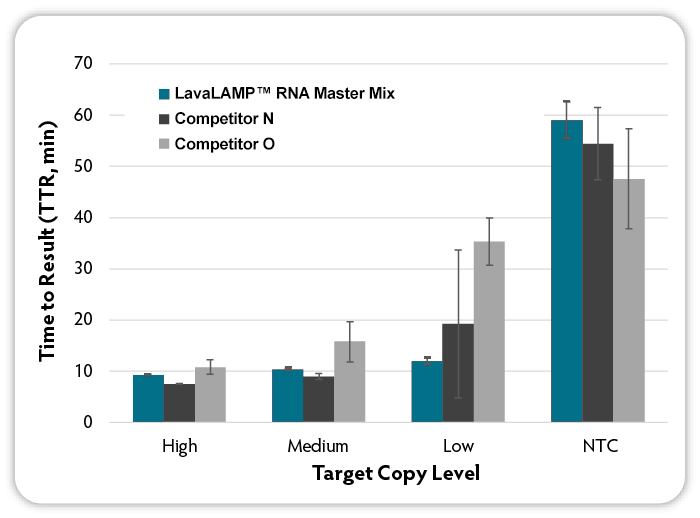
Figure 2. Loop-mediated isothermal amplification (LAMP) with real-time fluorescent detection of amplified products. RNA LAMP reactions (6 replicates per condition) were set up using the indicated kits according to manufacturer’s recommendations. Target RNA (MS2 bacteriophage) at varying input amounts, MS2-specific RNA LAMP primers, and Green Fluorescent Dye (LavaLAMP RNA Master Mix Kit) were included in all reactions. Reactions were run on a CFX96 Thermal Cycler (Bio-Rad) at the following temperatures: LavaLAMP; 68 °C; other kits at the recommended 65°C and fluorescence was measured over 60 minutes to determine the TTR. NTC denotes No Target Control.
Faster Zika Virus RNA Detection with LavaLAMP RNA Master Mix
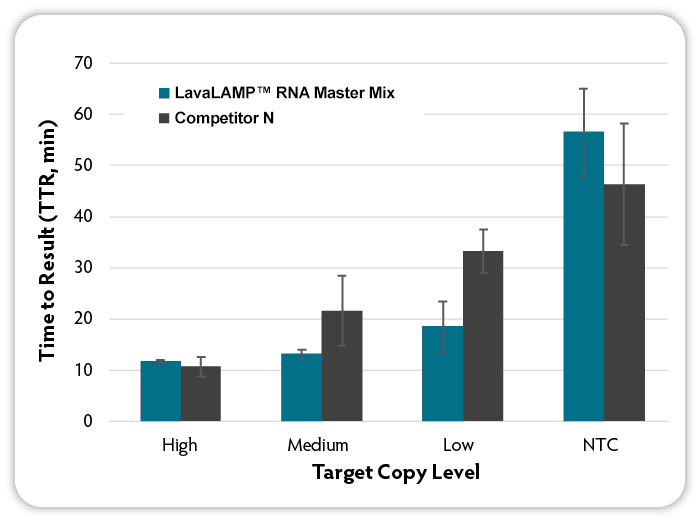
Figure 3: RNA LAMP-based detection of Zika Virus RNA. RNA LAMP reactions (6 replicates per condition) were set up using the indicated kits according to manufacturer’s recommendations. Target Zika virus RNA at varying input amounts, Zika-specific RNA LAMP primers, and Green Fluorescent Dye (LavaLAMP RNA Master Mix Kit) were included in all reactions. Reactions were run on a CFX96 Thermal Cycler (Bio-Rad) at the following temperatures: LavaLAMP; 68 °C; WarmStart® LAMP Kit; 65 °C, and fluorescence was measured over 60 minutes to determine the TTR. NTC denotes No Target Control.
The LavaLAMP RNA Master Mix is Optimised for RNA Detection
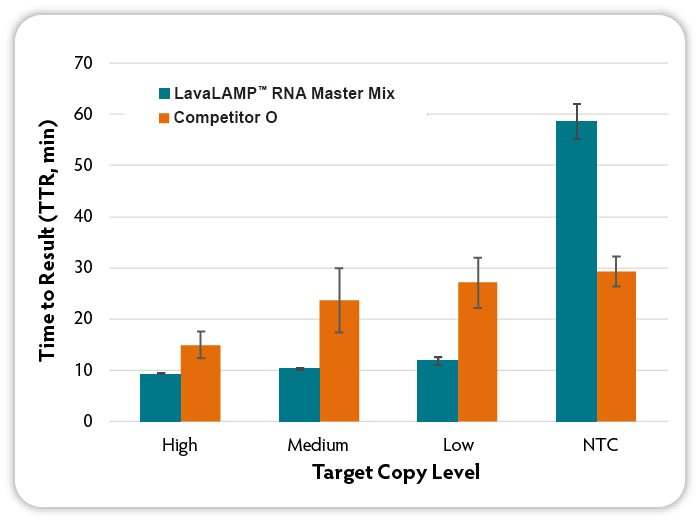
Figure 4: Comparing the performance of the LavaLAMP RNA Master Mix to the OmniAmp RNA & DNA LAMP Kit. RNA LAMP reactions (6 replicates per condition) were set up using the indicated kits according to manufacturer’s recommendations. For the OmniAmp® Kit, the final reactions had the following composition: 1X OmniAmp Buffer, 0.8 mM dNTPs, 8 mM MgSO4, 150 mM betaine and 1X OmniAmp Enzyme. The Target RNA (MS2 bacteriophage) at varying input amounts, and MS2-specific RNA LAMP primers, and Green Fluorescent Dye (LavaLAMP RNA Master Mix Kit) at the same amounts were included in all reactions. Reactions were run on a CFX96 Thermal Cycler (Bio-Rad) at 68 °C and fluorescence was measured over 60 minutes to determine the TTR. NTC denotes No Target Control.
The LavaLAMP RNA Master Mix is Compatible with Lyophilisation without Changing the Formulation
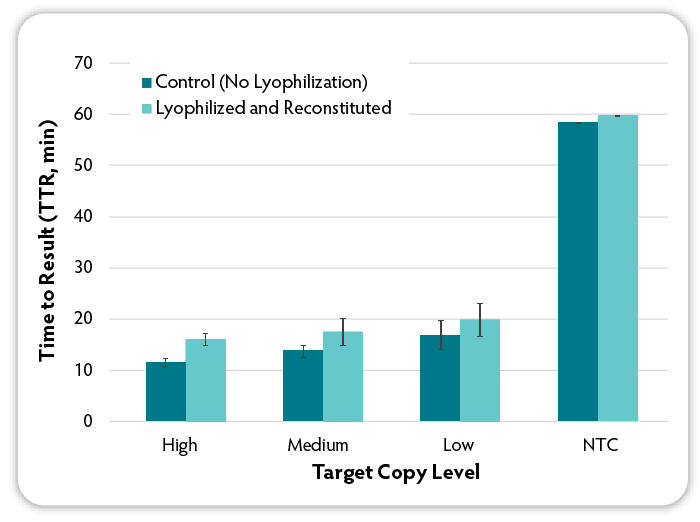
Figure 5: Testing the performance of lyophilised and then reconstituted LavaLAMP RNA Master Mix. The LavaLAMP RNA Master Mix was aliquoted (12.5 µL) into tubes and lyophilised using a VirTis Wizard 2.0. After one day, each lyophilised tube was reconstituted in 12.5 µL water. Then six RNA LAMP reactions were set up using the reconstituted lyophilised master mix and the standard LavaLAMP RNA Master Mix (Control) per test condition. Three levels of target MS2 RNA and identical amounts, MS2-specific RNA LAMP primers and Green Fluorescent Dye were added to the reactions. All reactions were incubated for 60 minutes in a CFX96 Thermal Cycler (Bio-Rad) at 68 °C and fluorescence was measured during the course of the reactions to determine the TTR. NTC denotes No Target Control.
Consistent Assay Performance Day to Day
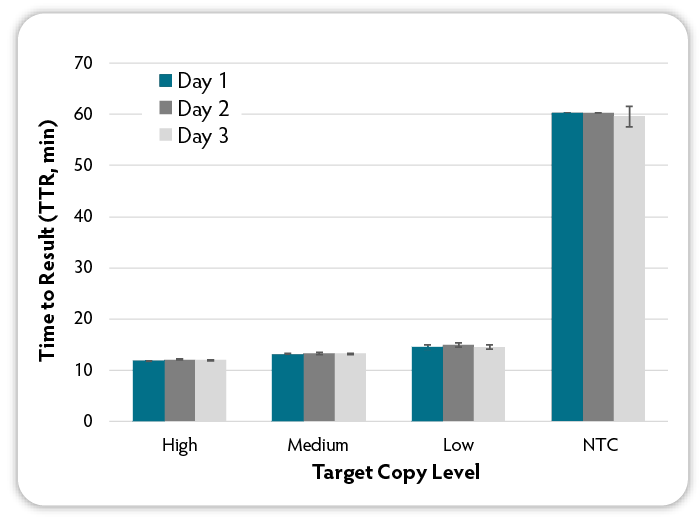
Figure 6: Analysis of day to day variability. On three different days, six replicate LAMP reactions were set up per condition using the indicated amounts of target RNA (MS2 bacteriophage) at varying input amounts and MS2-specific RNA LAMP primers and Green Fluorescent Dye were included in all reactions at the same amounts. Reactions were run on a CFX96 Thermal Cycler (Bio-Rad) at 68 °C and fluorescence was measured over 60 minutes to determine the TTR. NTC denotes No Target Control.
Robust Performance across Multiple Operators
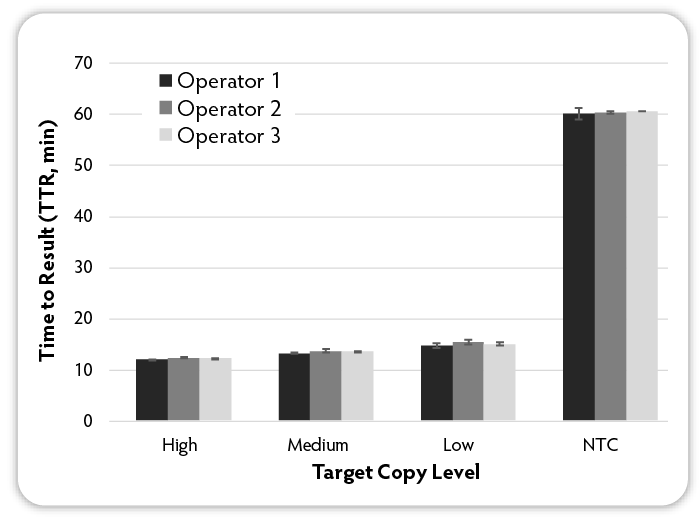
Figure 7: Analysis of operator to operator variability. Three different operators set up six replicate LAMP reactions per condition using the indicated amounts of target RNA (MS2 bacteriophage) at varying input amounts and MS2-specific RNA LAMP primers and Green Fluorescent Dye were included in all reactions at the same amounts. Reactions were run on a CFX96 Thermal Cycler (Bio-Rad) at 68 °C and fluorescence was measured over 60 minutes to determine the TTR. NTC denotes No Target Control.
Licensing information: Lucigen is a fully licensed provider of LAMP reagents for research use. Patents WO 00/28082, WO 01/34790, and WO 01/77317 regarding the LAMP method are owned by the Eiken Chemical Co. Ltd. LavaLAMP, OmniAmp® and Bst Polymerase, Exonuclease minus are sold by LGC, Biosearch Technologies under license for use in LAMP for research use only. The products may not be used for LAMP-based human or diagnostic purposes without obtaining a license from Eiken. US Patent 8093030 for LavaLAMP and OmniAmp is owned by LGC, Biosearch Technologies.
It is the sole responsibility of the buyer to ensure that use of the product does not infringe the patent rights of third parties. If the purchaser is not willing to accept these use limitations, LGC, Biosearch Technologies is willing to accept return of the product for a full refund.
SDS
-
30087 LavaLAMP RNA Master Mix with Dye
-
30086 LavaLAMP RNA Master Mix
-
EU IT 30087 1 LavaLAMP RNA Master Mix with Dye
-
EU IT 30086 1 LavaLAMP RNA Master Mix
Manuals and user guides
-
LavaLAMP RNA Master Mix
-
LavaLAMP RNA Master Mix Quick Protocol
-
LavaLAMP technical guide for new users
Product information sheets
Access support
Need some support with placing an order, setting up an account, or finding the right protocol?
Contact us